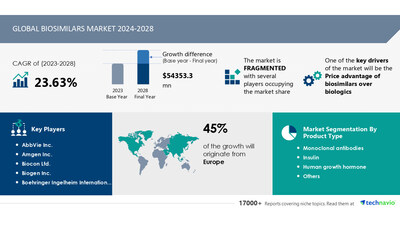
NEW YORK, Nov. 15, 2024 /PRNewswire/ — Report on how AI is redefining market landscape – The global biosimilars market size is estimated to grow by USD 54.35 billion from 2024-2028, according to Technavio. The market is estimated to grow at a CAGR of almost 23.63% during the forecast period. Price advantage of biosimilars over biologics is driving market growth, with a trend towards patients and physician’s willingness to switch to biosimilars. However, market access barriers for biosimilars poses a challenge.Key market players include AbbVie Inc., Amgen Inc., Biocon Ltd., Biogen Inc., Boehringer Ingelheim International GmbH, Celltrion Healthcare Co. Ltd., Dr Reddys Laboratories Ltd., F. Hoffmann La Roche Ltd., Fresenius SE and Co. KGaA, Gedeon Richter Plc, Halozyme Therapeutics Inc., Intas Pharmaceuticals Ltd., Mabion S.A., Novartis AG, Pfizer Inc., Samsung Biologics Co. Ltd., Sanofi SA, Teva Pharmaceutical Industries Ltd., and Viatris Inc..
AI-Powered Market Evolution Insights. Our comprehensive market report ready with the latest trends, growth opportunities, and strategic analysis- View Free Sample Report PDF
|
Forecast period |
2024-2028 |
|
Base Year |
2023 |
|
Historic Data |
2018 – 2022 |
|
Segment Covered |
Product Type (Monoclonal antibodies, Insulin, Human growth hormone, and Others), Application (Oncology and hematology, Endocrinology, Immunology, and Nephrology), and Geography (Europe, North America, Asia, and Rest of World (ROW)) |
|
Region Covered |
Europe, North America, Asia, and Rest of World (ROW) |
|
Key companies profiled |
AbbVie Inc., Amgen Inc., Biocon Ltd., Biogen Inc., Boehringer Ingelheim International GmbH, Celltrion Healthcare Co. Ltd., Dr Reddys Laboratories Ltd., F. Hoffmann La Roche Ltd., Fresenius SE and Co. KGaA, Gedeon Richter Plc, Halozyme Therapeutics Inc., Intas Pharmaceuticals Ltd., Mabion S.A., Novartis AG, Pfizer Inc., Samsung Biologics Co. Ltd., Sanofi SA, Teva Pharmaceutical Industries Ltd., and Viatris Inc. |
Key Market Trends Fueling Growth
The Biosimilars Market is experiencing significant growth, particularly in the treatment of chronic conditions such as diabetes, autoimmune diseases, and cancers. Active substances in biosimilars can come from various sources including human, animal, and microorganisms like bacteria and yeast. These substances often involve complex proteins like insulin, growth hormone, coagulation factors, and monoclonal antibodies. The European Union (EU) plays a crucial role in biosimilars through regulatory bodies like the European Medicines Agency (EMA) and the European Commission. Patients and healthcare professionals rely on these organizations to ensure the safety, efficacy, and interchangeability of biosimilars. The EU regulatory process for biosimilars includes a centralized procedure, clinical experience, and an assessment of the immunogenicity profile. Pharmaceutical quality, safety, and efficacy are top priorities. Biosimilars for conditions like rheumatic diseases are becoming increasingly important. To help understand this complex topic, the EU provides information guides and videos in various European languages. EU scientific experts collaborate with national health authorities and pharmacy levels for regulatory guidance and pharmacovigilance activities.
The biosimilar market’s growth is influenced by patient and physician attitudes towards biosimilar use. Many physicians, particularly in rheumatology, prefer prescribing bio-originators over biosimilars for treating rheumatology diseases. This resistance stems from the perception that biosimilars are less effective than their reference products. At the pharmacy level, biosimilar substitution is only permitted in certain countries, mainly in Central and Eastern Europe. Despite increasing patient and physician adoption, the rate remains low due to legislative restrictions.
Insights on how AI is driving innovation, efficiency, and market growth- Request Sample!
Market Challenges
- Biosimilars are becoming an essential part of the healthcare landscape, particularly in managing chronic conditions such as diabetes, autoimmune diseases, cancers, and more. However, producing biosimilars presents unique challenges. These include the use of active substances derived from human, animal, or microorganism sources like bacteria or yeast, and the complexity of living cells and organisms. The European Union (EU) and its regulatory bodies, including the European Medicines Agency (EMA) and the European Commission, play a crucial role in ensuring the safety, efficacy, and interchangeability of biosimilars. This involves a centralized procedure for marketing authorization, clinical experience, and an immunogenicity profile assessment. Patients and healthcare professionals, including Chief Medical Officers, rely on clear and accessible information. The EU provides an information guide for patients and healthcare professionals in various European languages. Pharmaceutical quality, safety, and efficacy are paramount, with regulatory guidance and pharmacovigilance activities ensuring these standards are met. Biosimilars cover a range of therapeutic areas, from insulin and growth hormone to coagulation factors and monoclonal antibodies. Challenges include molecular micro-heterogeneity, which requires careful consideration during the development process. National health authorities and Member States also play a role in the approval and implementation of biosimilars at the pharmacy level. Rheumatic diseases are just one of the many areas where biosimilars have shown promise. As the use of biosimilars continues to grow, it’s essential to stay informed about the latest regulatory developments and scientific advancements. For a more in-depth understanding, check out the EMA’s informative video on biosimilars.
- Biosimilars face significant challenges in competing with biologics and entering the market. The manufacturing process for biosimilars is intricate, requiring strict uniformity between batches. Variations are common, making consistency a major hurdle. This complexity is a significant challenge for new companies, while experienced biologics manufacturers will continue to dominate. Additionally, legal issues surround the approval process for biologics, adding another layer of complexity for biosimilar entrants.
Insights into how AI is reshaping industries and driving growth- Download a Sample Report
Segment Overview
This biosimilars market report extensively covers market segmentation by
- Product Type
- 1.1 Monoclonal antibodies
- 1.2 Insulin
- 1.3 Human growth hormone
- 1.4 Others
- Application
- 2.1 Oncology and hematology
- 2.2 Endocrinology
- 2.3 Immunology
- 2.4 Nephrology
- Geography
- 3.1 Europe
- 3.2 North America
- 3.3 Asia
- 3.4 Rest of World (ROW)
1.1 Monoclonal antibodies- Biosimilar monoclonal antibodies (mAbs) are large, complex proteins used by the immune system to identify and neutralize foreign bodies. They are used for treating various cancers, such as breast cancer, and non-cancer diseases, like rheumatoid arthritis. The development of biosimilars for complex mAbs is accelerating market growth due to patent expiries, approvals, and increasing chronic disease prevalence. Challenges exist, but late-stage clinical trials are underway, and approvals are expected. Cancer treatments, such as trastuzumab, have revolutionized, but high costs restrict access. Biosimilars, like approved trastuzumab biosimilars in the EU and US, offer cost-effective alternatives, driving market growth.
Download complimentary Sample Report to gain insights into AI’s impact on market dynamics, emerging trends, and future opportunities- including forecast (2024-2028) and historic data (2018 – 2022)
Research Analysis
The Biosimilars market refers to the sector of the pharmaceutical industry focused on developing and manufacturing follow-on biological medicines. These medicines are designed to have similar active substances, biological source, and therapeutic effects as original biologics, but are not exact copies. Chronic conditions such as diabetes, autoimmune diseases, cancers, and rheumatic diseases are common indications for biosimilars. Active substances can be derived from human, animal, or microorganisms, including bacteria and yeast. Proteins like insulin, growth hormone, coagulation factors, and monoclonal antibodies are common active substances used in biosimilars. The European Union (EU) regulates biosimilars through a centralised procedure, ensuring clinical experience, immunogenicity profile, pharmaceutical quality, safety, and efficacy are comparable to the reference product. The EU provides an information guide for scientific experts to assess these aspects. Data exclusivity, interchangeability, and pharmacovigilance activities are also key considerations. National health authorities and regulatory guidance play a crucial role in the approval process. Biosimilars are an essential alternative to expensive original biologics, offering cost savings while maintaining therapeutic equivalence. For more information, watch our video or consult our infographic in various European languages.
Market Research Overview
The Biosimilars Market refers to the sector dedicated to the development, production, and distribution of biosimilar medicines. Biosimilars are biological equivalents of original biologic medicines, which are used to treat chronic conditions such as diabetes, autoimmune diseases, cancers, and others. These medicines contain active substances derived from living cells, organisms, or microorganisms, including human, animal, and bacterial sources. The European Union (EU) plays a significant role in the regulation of biosimilars through the European Medicines Agency (EMA) and the European Commission. Patients and healthcare professionals benefit from biosimilars as they offer cost savings without compromising safety, efficacy, interchangeability, and pharmaceutical quality. The EU regulatory framework includes a centralised procedure, clinical experience, immunogenicity profile, and pharmacovigilance activities. The EMA provides an information guide for patients and healthcare professionals in European languages. Biosimilars include insulin, growth hormone, coagulation factors, monoclonal antibodies, and other proteins. The development of biosimilars involves rigorous testing to ensure molecular micro-heterogeneity and regulatory compliance. National health authorities and regulatory guidance are essential for the successful implementation of biosimilars at the pharmacy level. Rheumatic diseases are among the conditions that can be treated with biosimilars. The EU scientific experts and Member States work together to ensure the safety and efficacy of biosimilars through ongoing pharmacovigilance activities. An infographic and video resources are available to help understand the biosimilars market.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
- Product Type
- Monoclonal Antibodies
- Insulin
- Human Growth Hormone
- Others
- Application
- Oncology And Hematology
- Endocrinology
- Immunology
- Nephrology
- Geography
- Europe
- North America
- Asia
- Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio’s report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio’s comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: [email protected]
Website: www.technavio.com/
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biosimilars-market-to-grow-by-usd-54-35-billion-2024-2028-cost-advantage-over-biologics-to-drive-revenue-report-with-the-ai-impact-on-market-trends—technavio-302306608.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biosimilars-market-to-grow-by-usd-54-35-billion-2024-2028-cost-advantage-over-biologics-to-drive-revenue-report-with-the-ai-impact-on-market-trends—technavio-302306608.html
SOURCE Technavio

Featured Image: Megapixl @ Foxaon