SHANGHAI and NANJING, China and SAN JOSE, Calif., Sept. 27, 2024 /PRNewswire/ — IASO Biotherapeutics (“IASO Bio”), a biopharmaceutical company dedicated to discovering, developing, manufacturing and commercializing innovative cell therapy and antibody products, today announced a poster presentation of the outcomes of relapsed/refractory multiple myeloma (R/RMM) patients with renal impairment (RI) treated with the fully human anti-BCMA CAR-T cell therapy Equecabtagene Autoleucel (Eque-cel, FucasoTM), from the pivotal phase 2 FUMANBA-1 study at the 2024 International Myeloma Society (IMS) Annual Meeting. Results indicate that Eque-cel achieve equivalent efficacy and safety for R/RMM patients with renal impairment and improve the renal function as well.
Abstract Number: P-100
Abstract Title: The Outcomes of R/R MM Patients with Renal Impairment (RI) Treated with Eque-cel in the Pivotal Phase 2 FUMANBA-1 Study
Presentation Date: September 26, 2024(UTC-3)
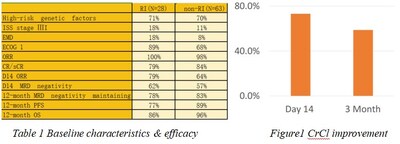
A total of 91 subjects, all without prior CAR-T treatment, were enrolled in the FUMANBA-1 study to receive Eque-cel, with a median follow-up of 18.07 months. Subjects were divided into RI group and non-RI group, based on creatinine clearance (CrCl) levels at the time of CAR-T therapy, of which 28 subjects had RI with CrCl between 40 and 70 ml/min, and 63 belonged to non-RI group, with CrCl>70 ml/min.
The baseline characteristics in the RI group were comparable to those in the non-RI group. The RI group achieved a response as rapid and deep as the non-RI group. Long-term efficacy in the RI group were not inferior (as shown in Figure1) as non-PI group. In terms of safety, both group developed cytokine release syndrome (CRS) Only one case of grade 2 immune effector cell-associated neurotoxicity syndrome (ICANS) was reported in the non-RI group, while no cases of ICANS occurred in the RI group. A slightly higher prevalence of short-term severe cytopenia was noted in the RI group, but recovered by day 60.
Conclusions: In the FUMANBA-1 study, R/RMM patients with RI could achieved a similarly rapid, deep, and durable response with Eque-cel treatment without compromising safety status. Additionally, renal function was improveddue to the clearance of myeloma cells by Eque-cel.
The principal investigator of this study, Professor Lugui Qiu, from the Institute of Hematology and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, and Professor Chunrui Li, from Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology, stated: “In the past, we were concerned about the potential intolerance of R/R MM patients with impaired renal function to CAR-T treatment. The current study confirmed that even in patients with renal impairment, Eque-cel can induce rapid, deep, and durable remission, with efficacy comparable to that of patients without renal impairment. This data provides invaluable insights for clinical treatment with Eque-cel for camplcated case.
Dr. Yongke Zhang, Chief Scientific Officer of IASO Bio, said: “We are pleased to present the retrospective study on the use of Eque-cel (FucasoTM) for treating R/RMM patients with renal impairment at the IMS Annual Meeting. Data from the FUMANBA-1 study demonstrated the promising efficacy and safety of Eque-cel in this patient population, with the added benefit of improving renal function. We believe CAR-T therapy will offer new therapeutic options for R/RMM patients with moderate to severe renal function impairment, enabling broader application in the field of myeloma treatment.”
About FUMANBA-1 Study
The FUMANBA-1 Study is a Phase Ib/II, single-arm, multicenter study to assess the efficacy and safety of the investigational drug Equecabtagene Autoleucel, a fully human BCMA CAR-T cell therapy, in patients with R/R MM who have received 3 or more lines of treatment.
About IASO Bio
IASO Bio is a biopharmaceutical company focused on the discovery and development of novel cell therapies and biologics for oncology and autoimmune diseases. IASO Bio possesses comprehensive capabilities spanning the entire drug development process, from early discovery to clinical development, regulatory approval, and commercialization.
Its pipeline includes a diversified portfolio of over 10 novel products, including Equecabtagene Autoleucel (a fully human BCMA CAR-T injection). Equecabtagene Autoleucel received Biologics License Application (BLA) approval from China’s National Medical Products Administration (NMPA) in June 2023 and U.S. FDA IND approval for the treatment of R/RMM in December 2022.
Leveraging its strong management team, innovative product pipeline, as well as integrated and high quality manufactural and clinical capabilities, IASO aims to deliver transformative, curable, and affordable therapies that fulfil unmet medical needs to patients in China and around the world. For more information, please visit http://www.iasobio.com or www.linkedin.com/company/iasobiotherapeutics.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/iaso-bio-presented-the-outcomes-of-relapsedrefractory-multiple-myeloma-rrmm-patients-with-renal-impairment-treated-with-equecabtagene-autoleucel-fucaso-at-2024-ims-annual-meeting-302260878.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/iaso-bio-presented-the-outcomes-of-relapsedrefractory-multiple-myeloma-rrmm-patients-with-renal-impairment-treated-with-equecabtagene-autoleucel-fucaso-at-2024-ims-annual-meeting-302260878.html
SOURCE IASO Bio

Featured Image: Megapixl @ Ipopba