Remember the shocking news of Black Panther star Chadwick Boseman’s tragic death from colon cancer?1
It wasn’t the fact that Boseman hid his illness from the world until his final days that stunned fans. It was because he wasn’t even 40 years old when he received the stage 3 diagnosis.
days that stunned fans. It was because he wasn’t even 40 years old when he received the stage 3 diagnosis.
Up until that point, most people thought colon cancer only affected people aged 55 and up, but the truth is colon cancer rates are rising dramatically among young and middle-aged adults.
What’s even scarier is that over one-third of those diagnosed will die.
The good news is that colon cancer is actually one of the most preventable with proper screening and we’ve discovered an under-the-radar small-cap stock that is dedicated to saving lives by transforming at-home cancer detection.
Breaking News
For a small-cap stock, getting approval from the Food and Drug Administration (FDA) for a drug or a diagnostic device can be the game changer that sends its value soaring. One stock investors should be watching closely right now is Mainz Biomed (NASDAQ:MYNZ).
At a market cap of only $80 million, it’s a small-sized biotech company with blue sky written all over it. The company has successfully completed its pre-submission process with the FDA for its PMA pivotal clinical trial for ColoAlert in 2023. That means that FDA approval could potentially be in the near future and if that happens, it could send this company into a new dimension.
To put it in perspective, despite generating over $2 billion in revenue, Mainz Biomed’s closest rival’s inferior product pales in comparison and their nearly $12 billion market cap can’t touch their game-changing innovation.
Plus, Mainz Biomed (NASDAQ:MYNZ) boasts a stacked roster of renowned scientists and oncology experts, many of whom have an impressive track record of experience at top companies like Roche, Abbott Laboratories, PharmGenomics, Deloitte, and Danaher, just to name a few.
6 Compelling Reasons
To Keep An Eye On Mainz Biomed (NASDAQ:MYNZ)
1
Commercially Ready Flagship Product: ColoAlert is a highly efficacious and easy-to-use at-home screening test for colorectal cancer (CRC) that’s the first DNA-based screening test for colorectal cancer that’s already approved in the EU. Now Mainz Biomed (NASDAQ:MYNZ) is currently in the process of seeking FDA approval in the US. Annual testing costs per patient are minimal, especially when compared to late-stage treatments of CRC, which cost patients an average of $38,469 per year.2
2
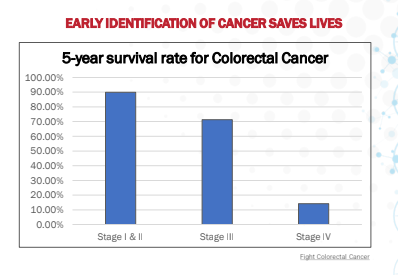
Significant Clinical Impact: The 5-year relative survival rate for colorectal cancer is approximately 90% if it is detected in its early stages, prior to spreading.3 In a multicentric study of 566 patients, ColoAlert showed the most accurate test results among non-invasive screening methods, with a sensitivity of 85% and a specificity of 92%.4 The Company is currently adding mRNA biomarkers to their test which have the potential to boost its clinical performance, especially at early stages.
3
Large Market: It’s estimated that the cancer screening market will be worth $281.4 billion by 2027,5 which is more than twice the value it was last year at $115.60 billion.6 In particular, the global non-invasive cancer diagnostics market is projected to reach $201 billion by 2027, up from $142 billion in 2023, growing at a CAGR of 7.2%.7
4
Differentiated Commercial Model: ColoAlert is already being commercialized across Europe through a differentiated business model of partnering with third-party laboratories for test kit processing versus the traditional methodology of operating a single facility. The Company expects to use this same model in the U.S. differentiating it from the competition, all of which follow a centralized model.
5
Pivotal FDA Study: Late in 2022, Mainz Biomed (NASDAQ:MYNZ) received approval from an independent Institutional Review Board (IRB) for the company’s US pivotal study to evaluate the clinical performance of ColoAlert, bringing the company closer to an FDA approval that would greatly open up the market potential for their flagship asset.
6
Led by Industry Experts with Significant Expertise: The Frankfurt-based company is led by a seasoned management team of global industry veterans supported by board members and scientific advisors including Dr. Heiner Dreismann, a former president and CEO at Roche Diagnostics.
Press Releases
- Mainz Biomed Processes First Patients From Colorectal Cancer Screening Campaign Through Its Corporate Health Portal In Partnership With Zöeller-Kipper
- Mainz Biomed Announces First Quarter 2023 Financial Results And Provides Corporate Update
- Mainz Biomed Partners With Microba Life Sciences For The Development Of PancAlert
- Mainz Biomed Announces Addition Of Eurofins GeLaMed To Its Growing Network Of Lab Partners
- Mainz Biomed Provides Full Year 2022 Financial Results
The Silent Killers: Colon and Pancreatic Cancer
Two of the most dangerous forms of cancer are those that afflict the colon and pancreas. One of the main problems with both of these cancers is the lack of screening options. Many cases go undetected until it is too late.
According to the American Cancer Society, about 1 in 23 men and 1 in 26 women will develop colorectal cancer during their lifetime.8 In Canada, it’s estimated that 1 in 16 men and 1 in 19 women will develop this type of cancer.9
Colon cancer is often considered a “silent” disease, meaning it usually has no symptoms. Symptoms that may occur include changes in bowel habits, abdominal pain, blood in the stool, and weight loss.
Meanwhile, pancreatic cancer is less common, as the 12th most common new cancer each year. Unfortunately, it is also the 4th leading cause of cancer deaths, after lung, colon, and breast cancers. This means that even though about 48,000 people are diagnosed each year, nearly the same number (40,000) will die from it.10
One of the biggest factors of success comes from early detection. What we know today though is that nearly half of all cancer deaths worldwide are preventable11 as many cancers are caused by preventable risk factors.12
What Boseman’s death in particular taught us was that colorectal cancer is being diagnosed more often among young and middle-aged adults.13 Recent studies show that screening colonoscopies can reduce the relative risk of getting colon cancer by 52% and the risk of dying from it by 62%.14
But colonoscopies are costing patients more and more, and many are looking towards more non-invasive screening methods, much like that of ColoAlert from Mainz Biomed (NASDAQ:MYNZ).
Mainz Biomed (NASDAQ:MYNZ) recently announced a research collaboration with Microba Life Sciences,15 a precision microbiome company using a world-leading technology platform to measure the human gut microbiome to discover and develop PancAlert, an early-stage disease screening test for pancreatic cancer.
Microba and Mainz Biomed will conduct a pilot research project using Microba’s proprietary metagenomic sequencing technology and bioinformatic techniques to potentially find novel microbiome biomarkers for pancreatic cancer detection as part of the collaboration.
The pilot project, which is projected to last until late 2023, will use Microba’s Community Profiler (MCP), Microba’s proprietary metagenomic platform technology. MCP has proven to be a best-in-class research technique, capable of producing comprehensive and accurate species profiles of human gastrointestinal samples.
Funding Pouring into the Early Detection Market
With its flagship asset ColoAlert, Mainz Biomed (NASDAQ:MYNZ) has solidified its position in a rapidly growing early detection market that’s not only becoming more and more profitable but also presenting an opportunity to save a huge amount of lives along the way.
Analysts are projecting that the cancer test market is going to get bigger and might be worth $261.34 billion by 2027. That’s more than twice as much as it was worth last year when it was $115.60 billion.16 Big biotech companies are buying up other companies to make their own products better and to use their technology.
In the colorectal screening market, the leading provider of cancer screening and diagnostic tests in the US is Exact Sciences17—which had its flagship screening asset ColoGuard approved by the FDA in 2014.18
Once that approval was issued, Exact Sciences was off to the races, growing from $400 million up to its current value of nearly $12 billion. The company also brought in over $2 billion in 2022,19 yet it’s only addressing 8% of the potential US market so far, leaving plenty of room for competition.
And that competition is coming, very, very soon…
Mainz Biomed’s (NASDAQ:MYNZ) flagship ColoAlert is already approved in the EU and poised to capture much of the same US market that ColoGuard has targeted. However, there’s a twist! MYNZ’s strategic rollout of ColoAlert involves changing the way the tests are sold.
So, pretty soon Mainz Biomed (NASDAQ:MYNZ) should have the ability (they have clearly demonstrated this) to reach the majority of the US market, just like it’s successfully been rolled out in Germany, once FDA approval comes—all while EXAS is only reaching that 8% previously mentioned with its centrally distributed business method.
ColoAlert is not only less expensive, but it’s also easier to administer than ColoGuard while being much more accurate than the standard Fecal Immunochemical Test (FIT), and much, much less invasive than a colonoscopy.20
Hologic, Inc. (NASDAQ:HOLX) generated $559.3M in diagnostics revenue in Q1 2023 alone and $332M for its breast screening product.21 Meanwhile, Guardant Health, Inc. (NASDAQ:GH) is also targeting early-stage colorectal, breast, and lung cancers with its product Guardant Reveal,22 and NanoString Technologies (NASDAQ:NSTG) with a series of its own oncology panels and assays.23
The market is also responding by throwing $300M at Freenome24 and $105M at Geneoscopy,25 both of which are making strides in the cancer screening market.
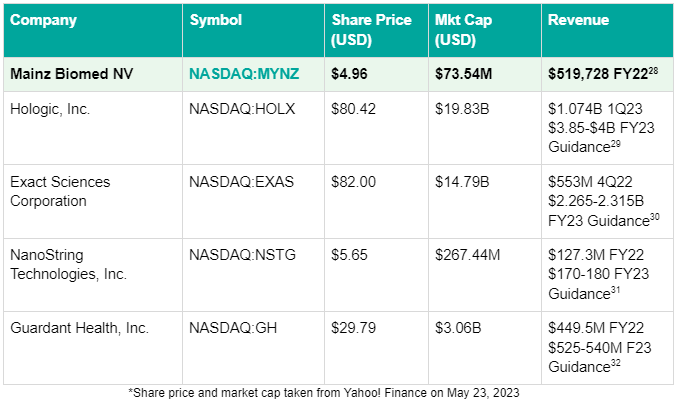
So in comparison, at a current market cap of only $88M, Mainz Biomed (NASDAQ:MYNZ) has plenty of potential value built in that could rapidly be unlocked should the FDA open the door to the US for ColoAlert with preparations for its pivotal FDA study now underway.26 The US market is clearly the crown jewel of the cancer screening market, and MYNZ is now waiting at the gates.
In the first quarter of 2023, ColoAlert® sales were $250,077, indicating a 152% increase over Q1 2022.27
ColoAlert is now being sold internationally through the Company’s unique business model of marketing products through partnerships with third-party laboratories rather than the traditional methodology of operating a single facility.
Mainz Biomed (NASDAQ:MYNZ) has developed a network of laboratory collaborations around Europe, with new additions in Germany, Spain, and England.
Looking into the space, it’s worth investigating how Mainz Biomed (NASDAQ:MYNZ) stacks up against other cancer screening tech stocks, and their revenue from cancer screening products:
Differentiated Business Model
Mainz Biomed (NASDAQ:MYNZ) is currently selling ColoAlert in Europe by working with other labs instead of having their own lab.
working with other labs instead of having their own lab.
The strategy involves giving ColoAlert to these labs, who can put their own name on it, and the labs buy the special kits from Mainz Biomed (NASDAQ:MYNZ) when they need them. Then, the labs can help doctors and patients find colorectal cancer early by using ColoAlert.
DNA extraction for ColoAlert is now automated on the Thermo ScientificTM Apex, representing an essential partnership that enables labs around the world to increase their testing capacity and optimize their resource allocation—and allowing for analyzing up to 96 samples per run, in roughly two hours.
On top of that, Mainz Biomed (NASDAQ:MYNZ) is also advancing its technology, having reported positive results from its Feasibility Study evaluating its portfolio of novel mRNA biomarkers for early detection of advanced colorectal adenomas.33

Mainz Biomed NV (NASDAQ:MYNZ) has added Eurofins GeLaMed to its network of lab partners offering PCR test kit processing of ColoAlert®, its flagship product that is a highly efficacious and simple to use at-home screening test for colorectal cancer (CRC).34 Eurofins GeLaMed has four facilities in Germany and is part of Eurofins Scientific, a multinational laboratory business with over 61,000 personnel in 61 countries and more than 450 million tests performed yearly.
Eurofins will offer ColoAlert at its testing facilities, and Mainz Biomed (NASDAQ:MYNZ) will assist with physician and patient education initiatives as part of the product introduction, emphasizing the necessity of early screening for the detection and prevention of CRC.
The Pivotal FDA Study
The FDA now thinks that people should get checked by screening once every three years when they turn 45 years old.35 In order to get there, the American Cancer Society recommends two categories of tests: stool-based tests, and visual tests.36
Among the easiest screening methods is Mainz Biomed NV’s (NASDAQ:MYNZ) ColoAlert, which has to-date delivered a 98% patient satisfaction rate37 and delivers up to 60% fewer missed cases compared to fecal immunochemical tests (FIT).38
But ColoAlert won’t be sold in the US until it receives proper FDA approval, and sure enough, MYNZ is on its way towards achieving that. Late in 2022, Mainz Biomed received approval from an independent Institutional Review Board (IRB) for the company’s US pivotal study to evaluate the clinical performance of ColoAlert, bringing Mainz Biomed (NASDAQ:MYNZ) closer to an FDA approval that would greatly open up the market potential for their flagship asset.39
ColoAlert is already approved in the EU, having achieved a new level of regulatory compliance in 2022.40 The product is CE-IVD marked (complying with EU safety, health, and environmental requirements) and is transitioning to compliance with In Vitro Diagnostic Devices Regulation (IVDR). It’s commercially available in a selection of countries in the European Union.
Now there’s an international clinical trial underway evaluating over 600 patients (women or men) in the age range of 40-85 at two participating centers in Norway and two in Germany.
As well, Mainz Biomed (NASDAQ:MYNZ) has announced its pivotal ReconAAsense study examining the clinical performance of mRNA and DNA test combined with a fecal immunochemical test for early detection of advanced adenoma and colorectal cancer to enroll 15,000 subjects across the US, with results expected in 2025.41
As well, Mainz Biomed (NASDAQ:MYNZ) is nearing completion of enrollment in its eAArly DETECT study, which is an extension of the ColoFuture feasibility study of a portfolio of novel gene expression (mRNA) biomarkers into ColoAlert.42 This study is expected to read out during 2023.
But it’s important to note that the ReconAAsense study in particular will form the basis of the data package for review by the US FDA to achieve marketing authorization.

Expert-Led Team
Leading the way for Mainz Biomed (NASDAQ:MYNZ) is a management team of industry experts with significant global expertise at top companies like Roche, Abbott Laboratories, PharmGenomics, Deloitte, and Danaher, fully equipped to bring the company and its flagship ColoAlert product toward commercial success.

6 Reasons
to Remember the names Mainz Biomed (NASDAQ:MYNZ) and ColoAlert Today!
1
Commercially Ready Product: ColoAlert is a highly efficacious and easy-to-use at-home screening test for colorectal cancer (CRC) that’s the first DNA-based screening test for colorectal cancer that’s already approved in the EU. Now Mainz Biomed (NASDAQ:MYNZ) is currently in the process of seeking FDA approval in the US.
2
Significant Clinical Impact: The 5-year relative survival rate for colorectal cancer is approximately 90% if it is detected in its early stages, prior to spreading.43 In a multicentric study of 566 patients, ColoAlert showed the most accurate test results among non-invasive screening methods, with a sensitivity of 85% and a specificity of 92%.44 Further, the clinical performance of its 2.0 product, currently in feasibility analysis in the eAArly Detect study, is expected to have potentially superior clinical performance.
3
Large Market: It’s estimated that the cancer screening market will be worth $281.4B by 2027,45 which is more than twice the value it was last year at $115.60B.46 In particular, the global non-invasive cancer diagnostics market is projected to reach $201B by 2027, up from $142B in 2023, growing at a CAGR of 7.2%.47
4
Differentiated Commercial Model: ColoAlert is already being commercialized across Europe and in select international markets through a differentiated business model of partnering with third-party laboratories for test kit processing versus the traditional methodology of operating a single facility. This model will be followed in the U.S., giving the Company access to the largest diagnostic lab networks in the U.S.
5
Pivotal FDA Study: Late in 2022, Mainz Biomed (NASDAQ:MYNZ) received approval from an independent Institutional Review Board (IRB) for the company’s US pivotal study to evaluate the clinical performance of ColoAlert, bringing the company closer to an FDA approval that would greatly open up the market potential for their flagship asset.
6
Led by Industry Experts with Significant Expertise: The Frankfurt-based company is led by a seasoned management team of global industry veterans supported by board members and scientific advisors.
With EU approval and sales already underway in that market Mainz Biomed (NASDAQ:MYNZ) is poised for big things in the global cancer detection market, as we await the results of their FDA clinical study, due in 2025.
With these studies already underway, you can expect MYNZ and ColoAlert news flow over the next few months to come very fast, with the potential for many of the same game-changing developments and milestones witnessed in the ColoGuard story.
So stay up to date on the story, by visiting the official Mainz Biomed (NASDAQ:MYNZ) website to learn more and subscribe for their news as it happens.








 Guido BaechlerCEO and Director
Guido BaechlerCEO and Director Dr. Heiner Dreismann, PhDChairman of the Board
Dr. Heiner Dreismann, PhDChairman of the Board William CaragolCFO
William CaragolCFO Dr. Moritz Eidens, PhDCSO and Director
Dr. Moritz Eidens, PhDCSO and Director Darin LeighChief Commercial Officer
Darin LeighChief Commercial Officer





